Home page
Welcome to our Team website
The Plant-Fusarium Interactions Research Group (led by Marc Somssich) is part of the School of BioSciences at the University of Melbourne, Australia.
Our Team is funded by a DECRA grant from the Australian Research Council, with previous support from the German Research Foundation through a postdoctoral research fellowship, and seed funds from the Melbourne Botany Foundation and an Early Career Research Grant from the University of Melbourne.
We are interested in the Plant Cell Wall as the first defense barrier against fungal pathogens. Our work is focused on the plants Arabidopsis thaliana and Brassica napus (canola), as well as the fungal pathogen Fusarium oxysporum. For our work we employ modern fluorescence microscopy, especially a custom-built vertical-stage microscope setup which allows us to grow plants and fungi directly on the microscope stage while being imaged.
We have received funding from:
-
Two new preprints online!
We finished 2022 with the publication of two new preprints, covering our Covid-19 pandemic-lockdown work. PREPRINT #1: First, we published our GreenGate vector set with 75 promoters of the plant immune system: "pGG-PIP: A GreenGate (GG) entry vector collection with Plant Immune system Promoters (PIP)" https://www.biorxiv.org/content/10.1101/2022.12.20.521163v1 We hope that this resource will be helpful to the community. For a thread covering the highlights of the preprints, …
20 January, 2023 -
New review on the A. thaliana – F. oxysporum 5176 pathosystem out
Our comprehensive overview article on the Arabidopsis thaliana - Fusarium oxysporum strain 5176 pathosystem is now published in the Journal of Experimental Botany: https://doi.org/10.1093/jxb/erac263 We have compiled pretty much all the data available for this pathosystem. Here's the index of what is covered: The role of phytohormones Salicylic acid Jasmonic acid Ethylene Abscisic acid Auxin The plant’s Fo5176 detection system WAKs and …
21 June, 2022 -
New Overview Article on “The Dawn of Plant Molecular Biology”
Here's a new overview article I have written during the Omicron lockdown in January: https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.417 I write about "The Dawn of Plant Molecular Biology", and how the adoption of Arabidopsis thaliana as universal plant model, the development of plant transformation, and the description of the CaMV 35S promoter started a molecular biology revolution in the plant sciences.
23 April, 2022 -
Back to work – After a world record 262 days in lockdown!
After a world record 262 days in lockdown, we are finally allowed to work again. Let's see if it really is for good this time. I sure hope so.
4 November, 2021 -
Lockdown number 6 continues
With the government acknowledging that they won't be able to get the current outbreak under control, we will remain in some form of lockdown until 85% of Victorians are vaccinated (~early December). Not looking good.
15 September, 2021 -
200 Days of Lockdown
Today we have reached 200 days of lockdown in Melbourne. And these 200 days are only the days of actual lockdown, meaning those of strict stay-at-home order. So it doesn't count all the weeks of gradual return to campus which followed every one of the five lockdowns we've had, where we are only allowed to work 1, 2 or 3 …
19 August, 2021 -
Lockdown number 6
Last week we were finally back in the lab after lockdown 5, and could get our plants out and experiments going again. Well, today we could throw them right in the bin again, as we have just entered lockdown number 6. Scheduled for 7 days for now.
5 August, 2021 -
Lockdown number five
Since Friday, July 16th, we are in our fifth lockdown, once again not able to work in the lab. I really hope that this stops soon. Hopefully we'll be back on Wednesday, July 28th.
23 July, 2021
Marc Somssich is interested in signalling dynamics that govern plant development on an individual cell level, in an intact tissue and organ context. He started off by analysing and comparing differential peptide-receptor signalling dynamics involved in plant stem cell or defence pathways (CLAVATA-WUSCHEL or flagellin, respectively), and has since moved on to study signalling events involving the plant cell wall.
The plant cell wall has long been looked at as merely a physical barrier, forming a defensive wall and providing mechanical strength. However, the plant cell wall is also a sensory organ, directly involved in different signalling pathways, connecting the plant cells with each other and their environment. Marc has studied the cell wall’s role in guiding gravitropic growth, and in response to abiotic (saline) stress, and is now focusing on biotic stress. More precisely, he is investigating the plant cell wall as a sensory organ involved in defence signalling events combating infection by the pathogenic fungus Fusarium oxysporum.
Since joining the University of Melbourne, I, err, I mean, Marc has developed a microscopy-based system that allows the live-imaging of the plant’s defence responses, be it changes in gene expression or protein dynamics and localisation, in real-time. These reactions of the plant immune system are typically recorded on a larger scale (whole plant, whole tissue, in vitro assays…), where the cellular details are often lost. The new system allows to distinguish between the responses of individual, neighbouring cells, thereby providing a much needed cellular resolution. Eventually the aim is to describe in detail how, for example, a cell responses that is under acute attack vs. a cell that is ‘warned’ by its neighbours of an impeding attack. Or a cell that is damaged vs. one that can already ‘sense’ the pathogen but is not damaged yet. Also, the system will be used to study the difference in response to a pathogenic F. oxysporum forma vs. a non-pathogenic forma.
More details and images to follow soon(ish).
The Plant-Fusarium Interactions Research Team:
Marc Somssich
 Marc studied biology at the University of Düsseldorf, Germany, where he received his Diploma (Master’s equivalent, though better) and the title Doctor rerum naturalium (Dr. rer. nat.; PhD-equivalent, but much better). He conducted the work for both, his Diploma and Dr., in the Insitute for Developmental Genetics, headed by Prof. Rüdiger Simon, which scandalously does not feature an alumni section on it’s webpage. During his time in Düsseldorf he investigated interaction-dynamics between different receptor-like protein-kinases involved in stem cell homeostasis or pathogen-perception, respectively. For this, he employed a variety of FRET-based fluorescence microscopy techniques in living plant cells. Once he had answered all open questions in the plant stem cell field, Marc moved to the University of Melbourne to join the Plant Cell Biology lab of Prof. Staffan Persson. Equipped with his own funding from the German Research Foundation, he was interested in how the cell wall is remodeled in response to a cell transitioning through its different developmental stages, or in response to external stimuli, such as gravitropism, soil salinity or a pathogenic attack. While the ‘developmental stages’ part of the project (the main part) unfortunately proved very problematic, the ‘gravitropism’ part resulted in a publication, the ‘soil salinity’ part in a PhD-position (see Liu’s work, below), and the ‘pathogen’ part resulted in the formation of the Plant-Fusarium Interactions Research Team (who’s webpage you are viewing right now) via a DECRA grant from the Australian Research Council.
Marc studied biology at the University of Düsseldorf, Germany, where he received his Diploma (Master’s equivalent, though better) and the title Doctor rerum naturalium (Dr. rer. nat.; PhD-equivalent, but much better). He conducted the work for both, his Diploma and Dr., in the Insitute for Developmental Genetics, headed by Prof. Rüdiger Simon, which scandalously does not feature an alumni section on it’s webpage. During his time in Düsseldorf he investigated interaction-dynamics between different receptor-like protein-kinases involved in stem cell homeostasis or pathogen-perception, respectively. For this, he employed a variety of FRET-based fluorescence microscopy techniques in living plant cells. Once he had answered all open questions in the plant stem cell field, Marc moved to the University of Melbourne to join the Plant Cell Biology lab of Prof. Staffan Persson. Equipped with his own funding from the German Research Foundation, he was interested in how the cell wall is remodeled in response to a cell transitioning through its different developmental stages, or in response to external stimuli, such as gravitropism, soil salinity or a pathogenic attack. While the ‘developmental stages’ part of the project (the main part) unfortunately proved very problematic, the ‘gravitropism’ part resulted in a publication, the ‘soil salinity’ part in a PhD-position (see Liu’s work, below), and the ‘pathogen’ part resulted in the formation of the Plant-Fusarium Interactions Research Team (who’s webpage you are viewing right now) via a DECRA grant from the Australian Research Council.
Marc is an Author for the Company of Biologist’s preLights Team and Assistant Features Editor for the journal Plant Physiology
Marc @ Google Scholar: https://scholar.google.com.au/citations?user=-YhIzdMAAAAJ&hl=en
Marc @ Twitter: https://twitter.com/somssichm
Marc @ Mastodon: @somssich@sciencemastodon.com
Marc @ ORCID: https://orcid.org/0000-0001-5092-6168
Marc @ ResearchGate: https://www.researchgate.net/profile/Marc_Somssich
Liu Wang
 Liu studied food biotechnology at China Agricultural University in Beijing, China. For her Master’s thesis in the lab of A/Prof. Lin Shen she studied the drought stress response in tomato, establishing CRISPR-Cas9-mediated genome editing of tomato in her lab. Feeling the pressure to publish a first-author paper from her thesis, she somewhat overshot the mark and published three within a year. Having generated CRISPR-Cas9-edited tomato lines for different MAPKs to build the lab’s future research on, co-authorship on several future papers was secured and she therefore decided to move on. After receiving a PhD-scholarship from the Chinese Scholarship Council, she joined Prof. Staffan Persson’s Plant Cell Biology lab at the University of Melbourne to study salinity tolerance in plants, thereby building on her experience studying drought tolerance, but switching from the cool tomato plant to the general model Arabidopsis thaliana. Taking over a project from Marc, and being co-supervised by Staffan and Marc, she was automatically usurped by the newly formed Plant-Fusarium Interactions Research Team. For her work, Liu focuses on the role of the COMPANION OF CELLULOSE SYNTHASE 1 (CC1) protein in maintaining cell wall synthesis under salt stress, and how it connects cellulose synthesis activity to cytoskeleton-dynamics. Furthermore, she has quickly become the resident expert for protein-immunoprecipitation experiments with subsequent proteomic analyses, creating protein-protein-interaction and phosphoproteomic data resources for the lab, thereby enabling new student projects and once again ensuring her place on the author list of several upcoming papers.
Liu studied food biotechnology at China Agricultural University in Beijing, China. For her Master’s thesis in the lab of A/Prof. Lin Shen she studied the drought stress response in tomato, establishing CRISPR-Cas9-mediated genome editing of tomato in her lab. Feeling the pressure to publish a first-author paper from her thesis, she somewhat overshot the mark and published three within a year. Having generated CRISPR-Cas9-edited tomato lines for different MAPKs to build the lab’s future research on, co-authorship on several future papers was secured and she therefore decided to move on. After receiving a PhD-scholarship from the Chinese Scholarship Council, she joined Prof. Staffan Persson’s Plant Cell Biology lab at the University of Melbourne to study salinity tolerance in plants, thereby building on her experience studying drought tolerance, but switching from the cool tomato plant to the general model Arabidopsis thaliana. Taking over a project from Marc, and being co-supervised by Staffan and Marc, she was automatically usurped by the newly formed Plant-Fusarium Interactions Research Team. For her work, Liu focuses on the role of the COMPANION OF CELLULOSE SYNTHASE 1 (CC1) protein in maintaining cell wall synthesis under salt stress, and how it connects cellulose synthesis activity to cytoskeleton-dynamics. Furthermore, she has quickly become the resident expert for protein-immunoprecipitation experiments with subsequent proteomic analyses, creating protein-protein-interaction and phosphoproteomic data resources for the lab, thereby enabling new student projects and once again ensuring her place on the author list of several upcoming papers.
Liu @ Google Scholar: https://scholar.google.com/citations?user=W209SQoAAAAJ&hl=en&oi=sra
Liu @ Twitter: https://twitter.com/Liu_Wang1
Jacob Calabria
 Jacob studied genetics for his bachelor’s degree at the University of Melbourne, and subsequently completed his Master’s thesis in the Drosophila lab of Dr. Michael Murray. For his project he identified genes regulating the mesenchymal to epithelial cell transition during development of the Drosophila melanogaster embryo midgut, as this cell transition is important not only for normal embryo development, but also has relevance for tissue repair and cancer metastasis. Following his time in the genetics department, Jacob assumed the role of a research technician for both, Prof. Staffan Persson’s Plant Cell Biology lab and Dr. Berit Ebert’s Plant Glycobiology lab, in order to gain more practical experience working in a molecular biology lab. He quickly earned a reputation as a highly skilled, talented and motivated technician, thereby qualifying to also work for Marc’s Plant-Fusarium Interactions Research Team. As part of the Team, one of Jacob’s main objectives is to establish a transformation protocol for canola, in order to obtain fluorescent marker lines.
Jacob studied genetics for his bachelor’s degree at the University of Melbourne, and subsequently completed his Master’s thesis in the Drosophila lab of Dr. Michael Murray. For his project he identified genes regulating the mesenchymal to epithelial cell transition during development of the Drosophila melanogaster embryo midgut, as this cell transition is important not only for normal embryo development, but also has relevance for tissue repair and cancer metastasis. Following his time in the genetics department, Jacob assumed the role of a research technician for both, Prof. Staffan Persson’s Plant Cell Biology lab and Dr. Berit Ebert’s Plant Glycobiology lab, in order to gain more practical experience working in a molecular biology lab. He quickly earned a reputation as a highly skilled, talented and motivated technician, thereby qualifying to also work for Marc’s Plant-Fusarium Interactions Research Team. As part of the Team, one of Jacob’s main objectives is to establish a transformation protocol for canola, in order to obtain fluorescent marker lines.
Hsiang-Wen Chen
 Hsiang-Wen is interested in plant movement, such as tropisms and nastic movements, but especially circumnutation, which is involved in shoot climbing and soil penetration of the root. Her fascination with this topic motivated her to pursue a career in plant science, when she studied Biology at National Taiwan University, where she completed her Master’s thesis in the lab of Asst.Prof. Shu-Jen Wang in the Department of Agronomy. For her thesis, she focused on root movements in Oryza sativa, studying the hormone signalling pathways that control the light-induced wavy-root morphology seen in some varieties, and how environmental stimuli alter circumnutation patterns of seminal roots. Following the completion of her work, she moved on to the lab of A/Prof. Shih-Long Tu at the Institute of Plant and Microbial Biology, Academia Sinica, to expand her skill set by working as a research assistant before starting her PhD-studies. With A/Prof. Tu she helped to identify molecular mechanisms of light-regulated alternative splicing in Physcomitrium patens (or, TMFKAPhyscomitrella patents) using RNAseq. At this point, with research experience in two different labs, two first-author publications and plenty of experience with a variety of molecular biology methods and techniques, Hsiang-Wen decided to pursue her PhD-studies in Prof. Staffan Persson’s Plant Cell Biology lab at the University of Melbourne, via the university’s Graduate Research Scholarship program. Here, she returned to her original research-interest of studying plant movement, by investigating the role of the microtubule-binding protein CELLULOSE-MICROTUBULE UNCOUPLING 1 (CMU1) in control cell wall integrity during root skewing and soil penetration. Since her work is heavily dependent on live cell microscopy, using Spinning Disc and AiryScan Laser Scanning confocal microscopes, Staffan and Hsiang-Wen recruited Marc as co-supervisor for her PhD, making her a member of the Plant-Fusarium Interactions Research Team.
Hsiang-Wen is interested in plant movement, such as tropisms and nastic movements, but especially circumnutation, which is involved in shoot climbing and soil penetration of the root. Her fascination with this topic motivated her to pursue a career in plant science, when she studied Biology at National Taiwan University, where she completed her Master’s thesis in the lab of Asst.Prof. Shu-Jen Wang in the Department of Agronomy. For her thesis, she focused on root movements in Oryza sativa, studying the hormone signalling pathways that control the light-induced wavy-root morphology seen in some varieties, and how environmental stimuli alter circumnutation patterns of seminal roots. Following the completion of her work, she moved on to the lab of A/Prof. Shih-Long Tu at the Institute of Plant and Microbial Biology, Academia Sinica, to expand her skill set by working as a research assistant before starting her PhD-studies. With A/Prof. Tu she helped to identify molecular mechanisms of light-regulated alternative splicing in Physcomitrium patens (or, TMFKAPhyscomitrella patents) using RNAseq. At this point, with research experience in two different labs, two first-author publications and plenty of experience with a variety of molecular biology methods and techniques, Hsiang-Wen decided to pursue her PhD-studies in Prof. Staffan Persson’s Plant Cell Biology lab at the University of Melbourne, via the university’s Graduate Research Scholarship program. Here, she returned to her original research-interest of studying plant movement, by investigating the role of the microtubule-binding protein CELLULOSE-MICROTUBULE UNCOUPLING 1 (CMU1) in control cell wall integrity during root skewing and soil penetration. Since her work is heavily dependent on live cell microscopy, using Spinning Disc and AiryScan Laser Scanning confocal microscopes, Staffan and Hsiang-Wen recruited Marc as co-supervisor for her PhD, making her a member of the Plant-Fusarium Interactions Research Team.
Hsiang-Wen @ Google Scholar: https://scholar.google.com/citations?user=N6-uOeAAAAAJ&hl=en
Hsiang-Wen @ Twitter: https://twitter.com/HsiangWen_Chen
Cooperations:
Staffan Persson
Our team originated from Staffan’s lab here at UniMelb, and Liu and Hsiang-Wen are co-supervised by Staffan, are working on projects from his lab, and are accordingly supported by him.
Alexander Idnurm
Since none of us are experts in Mycology, or know how to handle pathogenic fungi, we are lucky to have the Mycology Team of A/Prof. Alexander Idnurm housed in our School of BioSciences building. Alex and his team are not just general experts for fungi, but are a leading lab for plant pathogenic fungi. Their main focus lies on Leptosphaeria maculans, the causal agent of blackleg disease in canola, for our joined project, however, they are more than happy to work with Fusarium oxysporum and have already created a whole suit of fluorescent marker lines for us to work with.
Biological Optical Microscopy Platform (BOMP)
A big part of the work in our team is based on fluorescent microscopy. Accordingly, BOMP is an important partner for us at the University. They provide us with modern, serviced microscopes, as well as image analysis software, know-how, trainings and workshops.
Google Scholar Publication lists for
Marc Somssich: https://scholar.google.com.au/citations?user=-YhIzdMAAAAJ&hl=en
Liu Wang: https://scholar.google.com/citations?user=W209SQoAAAAJ&hl=en&oi=sra
Jacob Calabria:
Hsiang-Wen Chen: https://scholar.google.com/citations?user=N6-uOeAAAAAJ&hl=en
Publications:
Calabria, J., Wang, L., Rast-Somssich, M. I., Chen, H.-W., Watt, M., Persson, S., Idnurm, A., Somssich, M. (2022). Spatially distinct phytohormone responses of individual Arabidopsis thaliana root cells to infection and colonization by Fusarium oxysporum. bioRxiv.: 521292. https://doi.org/10.1101/2022.12.20.521292
Calabria, J.,Rast-Somssich, M. I., Wang, L., Chen, H.-W., Watt, M., Idnurm, A., Persson, S., Somssich, M. (2022). pGG-PIP: A GreenGate (GG) entry vector collection with Plant Immune system Promoters (PIP). bioRxiv.: 521163. https://doi.org/10.1101/2022.12.20.521163
Wang, L., Calabria, J., Chen, H.-W., Somssich, M. (2022). Overview: The Arabidopsis thaliana – Fusarium oxysporum strain 5176 pathosystem. J. Exp. Bot.: erac263. https://doi.org/10.1093/jxb/erac263
Somssich, M. (2022). The Dawn of Plant Molecular Biology: How Three Key Methodologies Paved the Way. Current Protocols: 417. https://doi.org/10.1002/cpz1.417
Somssich, M. (2021). A short history of Plant Light Microscopy. Zenodo: 1-40. https://doi.org/10.5281/zenodo.4682572
Qiao, Z., Lampugnani, E. R., Yan, X.-F., Khan, G. A., Saw, W. G., Hannah, P., Qian, F., Calabria, J., et al. (2021). Structure of Arabidopsis CESA3 catalytic domain with its substrate UDP-glucose provides insight into the mechanism of cellulose synthesis. PNAS: https://doi.org/10.1073/pnas.2024015118
Somssich, M., Vandenbussche, F., Ivakov, A., Funke, N., Ruprecht, C., Vissenberg, K., et al. (2021). Brassinosteroids Influence Arabidopsis Hypocotyl Graviresponses Through Changes In Mannans And Cellulose. Plant & Cell Physiology: https://doi.org/10.1093/pcp/pcab024 (previously on bioRxiv.: https://doi.org/10.1101/557777)
Wang, L., Lampugnani, E. R., Persson, S. (2021). Salt with a sweet-tooth: Galactan synthesis impacts salt tolerance in Arabidopsis.: https://doi.org/10.1016/j.molp.2021.01.011
Wang, L.*, Hart, B. E.*, Khan, G. A., Cruz, E. R., Persson, S., Wallace, I. S. (2020). Associations between phytohormones and cellulose biosynthesis in land plants.: https://doi.org/10.1093/aob/mcaa121 * = Equal contribution
Cesarino, I.*, Dello Ioio, R.*, Kirschner, G. K.*, Ogden, M. S.*, Picard, K. L.*, Rast-Somssich, M. I.*, and Somssich, M.*,# (2020). Plant Science’s Next Top Models. Annals of Botany.: https://doi.org/10.1093/aob/mcaa063 * = Equal Contribution ; # = Corresponding author
Somssich M. (2020) A short history of Vernalization. Zenodo: 1–28. https://doi.org/10.5281/zenodo.3660691
Somssich M. (2019) A short history of Plant Transformation. PeerJ Prepr.: 1–28. https://doi.org/10.7287/peerj.preprints.27556
Lampugnani, E. R.*, Wink, R. H.*, Persson, S. and Somssich, M.# (2018). The Toolbox to Study Protein–Protein Interactions in Plants. Crit. Rev. Plant. Sci. https://doi.org/10.1080/07352689.2018.1500136. * = Equal Contribution ; # = Corresponding author
Somssich M. (2018) A short history of the CaMV 35S Promoter. PeerJ Prepr.: 1–16. http://doi.org/10.7287/peerj.preprints.27096
Sakamoto S., Somssich M., Nakata M.T., Unda F., Atsuzawa K., Kaneko Y., et al. (2018). Complete substitution of a secondary cell wall with a primary cell wall in Arabidopsis. Nature Plants: https://doi.org/10.1038/s41477-018-0260-4.
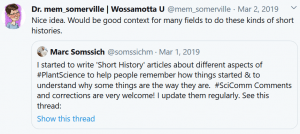
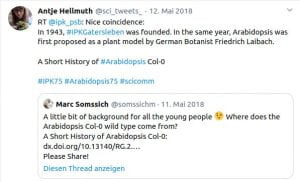
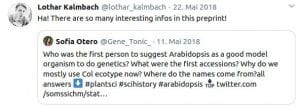
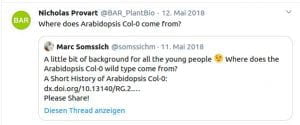
Somssich M. (2018) A short history of Arabidopsis thaliana (L.) Heynh. Columbia-0. PeerJ Prepr.: 1–7. http://doi.org/10.7287/peerj.preprints.26931
Lampugnani, E. R.*, Khan, G. A.*, Somssich, M.*, and Persson, S. (2018). Building a plant cell wall at a glance. J. Cell Sci. 131. https://doi.org/10.1242/jcs.207373. * = Equal Contribution
Brambilla, V., Martignago, D., Goretti, D., Cerise, M., Somssich, M., de Rosa, M., et al. (2017). Antagonistic Transcription Factor Complexes Modulate the Floral Transition in Rice. The Plant Cell 1, tpc.00645.2017. https://doi.org/10.1105/tpc.17.00645.
Somssich, M., and Simon, R. (2017). “Studying Protein–Protein Interactions In Planta Using Advanced Fluorescence Microscopy,” in Methods in Molecular Biology, ed. W. Busch (Springer Berlin Heidelberg), 267–285. https://doi.org/10.1007/978-1-4939-7003-2_17.
Breuer, D., Nowak, J., Ivakov, A., Somssich, M., Persson, S., and Nikoloski, Z. (2017). System-wide organization of actin cytoskeleton determines organelle transport in hypocotyl plant cells. Proc. Natl. Acad. Sci. U. S. A. https://doi.org/10.1073/pnas.1706711114.
Somssich, M., Je, B. Il, Simon, R., and Jackson, D. (2016). CLAVATA-WUSCHEL signaling in the shoot meristem. Development 143, 3238–3248. https://doi.org/10.1242/dev.133645
Somssich, M., Khan, G. A., and Persson, S. (2016). Cell Wall Heterogeneity in Root Development of Arabidopsis. Front. Plant Sci. 7, 1–11. https://doi.org/10.3389/fpls.2016.01242.
Liu, Z., Schneider, R., Kesten, C., Zhang, Y., Somssich, M., Zhang, Y., et al. (2016). Cellulose-Microtubule Uncoupling Proteins Prevent Lateral Displacement of Microtubules during Cellulose Synthesis in Arabidopsis. Dev. Cell 38, 305–15. https://doi.org/10.1016/j.devcel.2016.06.032.
Somssich, M., Bleckmann, A., and Simon, R. (2016). Shared and distinct functions of the pseudokinase CORYNE (CRN) in shoot and root stem cell maintenance of Arabidopsis. J. Exp. Bot. 67, 4901–4915. https://doi.org/10.1093/jxb/erw207.
Somssich, M., Ma, Q., Weidtkamp-Peters, S., Stahl, Y., Felekyan, S., Bleckmann, A., et al. (2015). Real-time dynamics of peptide ligand-dependent receptor complex formation in planta. Sci. Signal. 8, 1–9. https://doi.org/10.1126/scisignal.aab0598.
Lindner, M., Simonini, S., Kooiker, M., Gagliardini, V., Somssich, M., Hohenstatt, M. L., et al. (2013). TAF13 interacts with PRC2 members and is essential for Arabidopsis seed development. Dev. Biol. 379, 28–37. https://doi.org/10.1016/j.ydbio.2013.03.005.
Somssich, M.# , and Simon, R. (2012). “Peptides Regulating Apical Meristem Development,” in Signaling and Communication in Plants., eds. H. R. Irving and C. Gehring (Berlin, Heidelberg: Springer Berlin Heidelberg), 25–39. https://doi.org/10.1007/978-3-642-27603-3_2. # = Corresponding author
Following discussions I had with students (from undergrad to PhD-student level), I noticed that most young researchers were never taught anything about the historical context of our work. Context, however, still matters today. For example, many students are not aware of what the difference between the Arabidopsis lines Col-0, Col-1 and Landsberg is. Or why we are using the CaMV 35s promoter all the time, even though it is actually quite problematic to work with. For this reason, I have started to write ‘Short History’ articles on such aspects of plant science. To date, four chapters have been published:
Chapter 1 (2018): A short history of Arabidopsis thaliana (L.) Heynh. Columbia-0
PeerJ Prepr.: 1–7. http://doi.org/10.7287/peerj.preprints.26931
Chapter 2 (2018): A short history of the CaMV 35S Promoter
PeerJ Prepr.: 1–16. http://doi.org/10.7287/peerj.preprints.27096
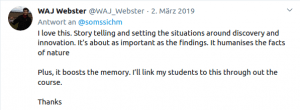
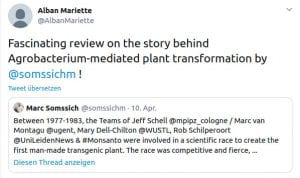
Chapter 3 (2019): A short history of Plant Transformation
PeerJ Prepr.: 1–28. https://doi.org/10.7287/peerj.preprints.27556
-> A compilation of chapters 1 – 3 has now been peer-reviewed and published as
Somssich, M. (2022). The Dawn of Plant Molecular Biology: How Three Key Methodologies Paved the Way
Current Protocols: 417. https://doi.org/10.1002/cpz1.417
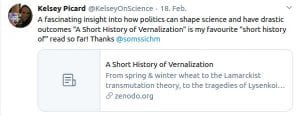
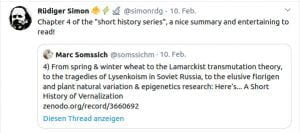
Chapter 4 (2020): A short history of Vernalization
Zenodo: 1–29 https://doi.org/10.5281/zenodo.3660691
Chapter 5 (2021): A short history of Plant Light Microscopy
Zenodo: 1-40 https://doi.org/10.5281/zenodo.4682572
-> Chapter 5 has now been peer-reviewed and published as
Somssich, M. (2022). A Short History of Plant Light Microscopy
Current Protocols: 577. https://doi.org/10.1002/cpz1.577
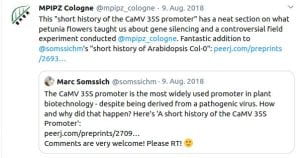
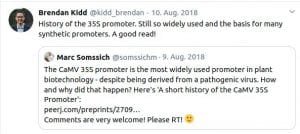
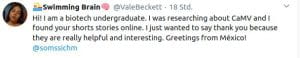
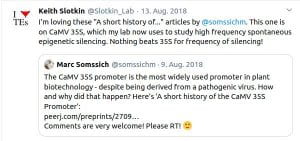
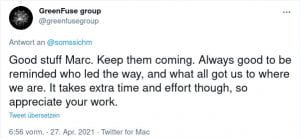
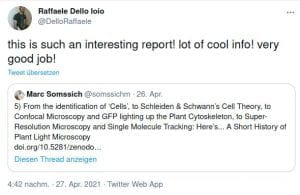
Some Online Feedback on the Short History articles (click to enlarge):
Address:
Marc Somssich
BioSciences 2, Room 301
School of BioSciences
University of Melbourne
Parkville, 3010, Victoria
Australia
Email (…unimelb.edu.au):
Marc: marc.somssich@ (…ending above)
Liu: wlw@student. (…ending above)
Jacob: calabria.j@ (…ending above)
Hsiang-Wen: hsiangc@student. (…ending above)
Twitter: